Advil PM Products
Put pain to rest and sleep the whole night with Advil PM. Non-habit forming Advil PM combines Ibuprofen with Diphenhydramine to help you sleep the whole night.†
Advil PM Caplets
Advil PM relieves your pain so you can sleep the whole night and feel rested in the morning.
Advil PM Liqui-Gels
Rushes liquid fast pain relief to where it hurts to help you sleep the whole night†.
Compare Advil Products

Count 300
Form Tablets
Key feature Easy to swallow
Ingredient Ibuprofen
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage 1 tablet every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 tablets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 200
Form Capsules
Key feature Small and easy to swallow
Ingredient Liquid Ibuprofen
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage 1 capsule every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 capsules in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 160
Form Capsules
Key feature Fast-acting
Ingredient Liquid Ibuprofen
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage 1 capsule every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 capsules in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 200
Form Tablets
Key feature Easy open cap
Ingredient Ibuprofen
Fast-acting ✓
Duration Up to 6h
Dosage 1 tablet every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 tablets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 144ct
Form Caplet
Key feature Target Back Pain
Ingredient Ibuprofen + Acetaminophen
Fast-acting ✓
Duration Up to 8h
Dosage 2 caplets every 8 hours. Do not exceed 6 caplets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.
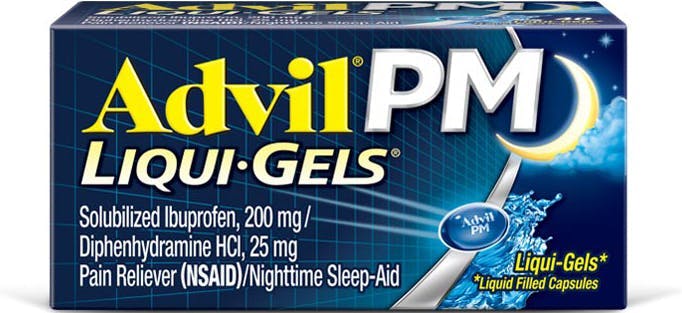
Count 80
Form Capsules
Key feature Fast-acting
Ingredient Sulobilized Ibuprofen / Diphenhydramine
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage Take 2 capsules at bedtime. Do not take more than 2 capsules in 24h.

Count 160
Form Capsules
Key feature Fast-acting
Ingredient Liquid Ibuprofen
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage 1 capsule every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 capsules in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.
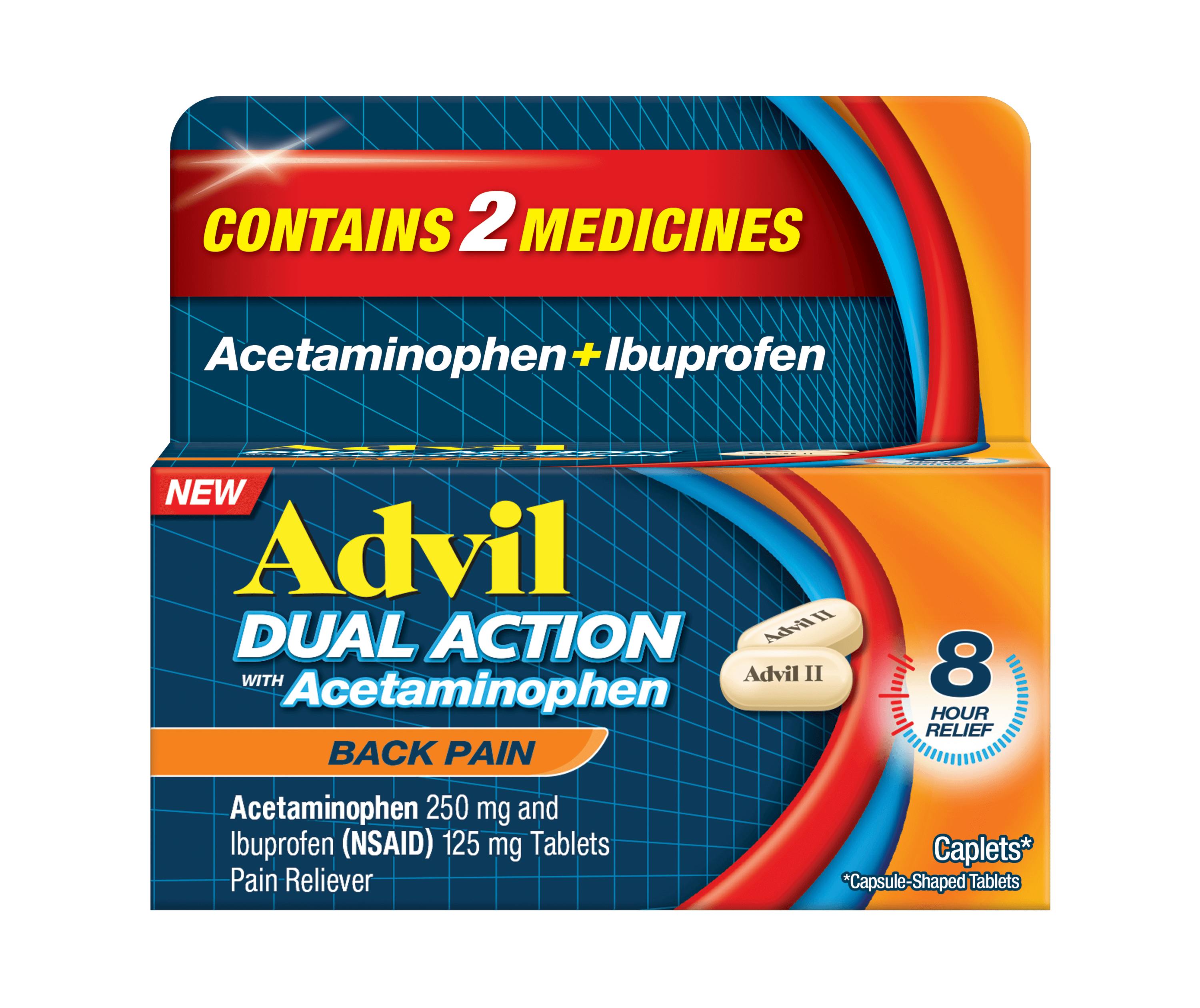
Count 144ct
Form Caplet
Key feature Targets Back Pain
Ingredient Ibuprofen + Acetaminophen
Fast-acting ✓
Duration Up to 8h
Dosage 2 caplets every 8 hours. Do not exceed 6 caplets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 200
Form Capsules
Key feature Small and easy to swallow
Ingredient Liquid Ibuprofen
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage 1 capsule every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 capsules in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 200
Form Tablets
Key feature Easy open cap
Ingredient Ibuprofen
Fast-acting ✓
Duration Up to 6h
Dosage 1 tablet every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 tablets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 144ct
Form Caplet
Key feature Target Back Pain
Ingredient Ibuprofen + Acetaminophen
Fast-acting ✓
Duration Up to 8h
Dosage 2 caplets every 8 hours. Do not exceed 6 caplets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 80
Form Capsules
Key feature Fast-acting
Ingredient Sulobilized Ibuprofen / Diphenhydramine
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage Take 2 capsules at bedtime. Do not take more than 2 capsules in 24h.

Count 160
Form Capsules
Key feature Fast-acting
Ingredient Liquid Ibuprofen
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage 1 capsule every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 capsules in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 300
Form Tablets
Key feature Easy to swallow
Ingredient Ibuprofen
Easy to swallow ✓
Fast-acting ✓
Duration Up to 6h
Dosage 1 tablet every 4 - 6 hours. If symptoms persist, 2 may be used. Do not exceed 6 tablets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.

Count 144ct
Form Caplet
Key feature Targets Back Pain
Ingredient Ibuprofen + Acetaminophen
Fast-acting ✓
Duration Up to 8h
Dosage 2 caplets every 8 hours. Do not exceed 6 caplets in 24h unless directed by a doctor. Under 12 y/o: Ask a doctor.